I am again indebted to my APDT students, who asked a very interesting question which turned into a blog post. This time it was: “Can a father’s stress be passed on epigenetically to his offspring through sperm?” Warning: epigenetic geekery ahead. If you are not in the mood for some technical terminology, this may not be the post for you.
I’ve blogged about epigenetics before (on the
epigenetics of fear and of
stress) and there are summaries of what epigenetics is in those two posts, but basically it’s changes in the DNA that don’t involve the sequence of bases. We’ve been so focused on the importance of DNA sequence as we’ve learned more and more about genetics, but in recent years the importance of other factors has started to become obvious. It’s like saying that the content of a book isn’t the only thing controlling whether the book gets read or not — it also matters whether the cover is appealing, how much it costs, and whether it’s shelved where people can find it.
One of my students tried to answer her own question and found this fascinating article:
Rodgers A.B., S. L. Bronson, S. Revello & T. L. Bale (2013). Paternal Stress Exposure Alters Sperm MicroRNA Content and Reprograms Offspring HPA Stress Axis Regulation, Journal of Neuroscience, 33 (21) 9003-9012. DOI: http://dx.doi.org/10.1523/jneurosci.0914-13.2013
Essentially, Rodgers et al stressed out some male mice and then tested their offspring six ways from Sunday to see if the effects had been passed on. And they had, but in some surprising ways.
The mice
Male mice were chosen because we have evidence that environmental effects can be passed on epigenetically through the sperm. Because sperm are made throughout the male’s lifetime, they can easily serve as messengers to pass information about the environment on from fathers to their offspring. Mothers can’t pass this information on through their eggs (so far as we know), because eggs are made before a female is born, and can’t easily be changed later. Of course, a mother has plenty of other chances to pass on information about the environment to her offspring: while they’re in utero and while they’re dependent on her. But for dad, sometimes he just has that one chance; he may never interact with his offspring in any other way than through the information in his sperm.
(Why do parents want to give their offspring information about the environment? To let them know, at formative times in their lives, how to develop. If the world is a safe one, you don’t need a highly reactive stress system. But if it’s a dangerous place, you need your store of cortisol ready to go. It’s easiest for these sorts of developmental decisions about how to tune the stress system to be made early in development — in utero or post-natally — so that’s why parents have systems to pass the information on early, early, early.)
Male mice, then, were chosen for this study. In addition to the control group of unstressed mice, there were two groups of stressed mice: mice who were stressed in adolescence, and mice who were stressed as adults. Epidemiological research in humans suggests that adolescence is an important time for epigenetic changes in sperm.
The stressors
The males were subjected to a variety of stressors. In reading the list, I was torn between sympathy for the mice, and bemusement at the entries:
Stressors, selected because they are nonhabituating, do not induce pain,
and do not affect food or water intake, included
the following: 36 h constant light, 15 min
exposure to fox odor,
novel object
(marbles) overnight, 15 min restraint in a
50 ml conical tube, multiple cage changes, novel 100 dB white noise
(Sleep Machine;
Brookstone) overnight, and saturated
bedding overnight.
Wet beds! Scary white noise! Scary marbles! And yet yes, probably very stressful to a mouse, and I should not make fun.
Breeding
The males were given time to recover from the stress and then bred. They were removed from the cage as soon as they had mated with the female, which took 1-3 days, to minimize their interactions with her, so that their stress levels could not affect her.
(However, the smart
reviewers at F1000 [warning: not open content] note that a stressed male might have been more aggressive in mating, which could cause the female to alter her care of her offspring.)
Offspring stress response
The offspring of stressed males, it turned out, had a
less responsive stress response than the offspring of unstressed males. In other words, when these mice were stressed by being restrained in a conical tube for 15 minutes, the ones whose fathers had undergone the variety of stressors had a smaller cortisol response compared to the ones whose fathers had not been stressed. The result was almost exactly the same whether the fathers had been stressed as adolescents or as adults, which surprised the researchers
.
Now, if a mouse receives information from his father (or his father’s sperm, but you know what I mean) that the world he’s going to live in is a stressful place, I would have expected that that mouse would develop a
more reactive stress system, not less. Worried that terrifying marbles or a wet bed are going to attack you at any moment? Then you had better have your stress response at the ready, right?
The stress system is, of course, much more complicated than that. We don’t understand yet why some models of stress system dysregulation show less reactive responses and some show more reactive responses. For example, humans with depression or PTSD can both show either more or less reactive stress responses than mentally healthy humans. So what exactly does this mean for these particular mice? The next thing
I would do is to look at their behavior. Do they
act more stressed?
Offspring behavior
The offspring were subjected to quite a few and quite varied tests to see if their stress behaviors were different. The researchers tested things like how much the offspring startled in response to a loud sound; how fearful they were of being in a brightly lit box versus a dark box (mice feel safer in darkness); how long they struggled when suspended by their tails; and more. Really surprisingly (to me, at least), there were no behavioral differences between the offspring of the stressed fathers and the offspring of unstressed fathers, despite this significant difference in stress system responsiveness. So what does
that mean?
The researchers tested a bunch of other stuff that left them empty handed as well, like gene expression differences in the brains and adrenals of the different sets of mice. All nothing. But what did they find that was different? They found an epigenetic difference in the fathers’ sperm.
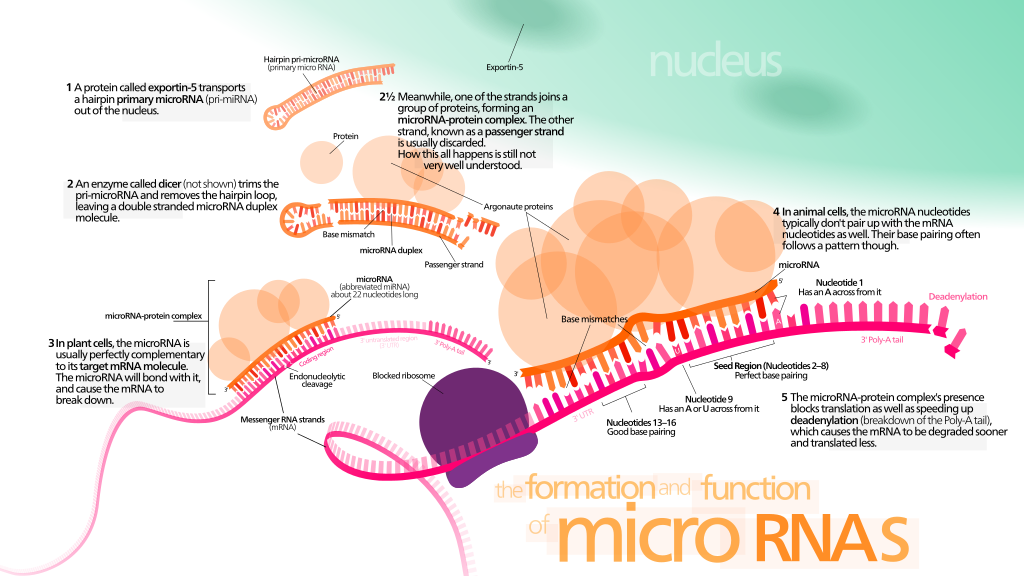
microRNA changes in sperm
Epigenetics is all about gene expression: determining which genes are used frequently to make their associated proteins, and which are left to lie dormant. The two best understood epigenetic mechanisms, acetylation and methylation, affect how much messenger RNA (mRNA) is transcribed from a particular gene. If there is more messenger RNA for a particular gene, then it’s easier to go the next step and make more of the protein that that gene codes for, and that gene’s expression increases. The mechanism that these researchers looked at is different. Instead of methylation and acetylation, they looked at microRNAs (
miRNA) in the sperm of these mice. Where methylation and acetylation affect how much messenger RNA is generated, microRNAs attach to the messenger RNA itself after it has been created, and silence it.
The way it works is this: since RNA is basically half of a double strand of DNA, it’s really sticky. It wants to find something that looks like its complement and stick to it. So microRNAs are little bits of RNA that stick to particular messenger RNAs. Then when the cell takes those messenger RNAs and tries to use them to make a protein — it can’t. Because there’s this microRNA stuck to it, blocking the sequence of the message. So microRNAs reduce the expression of a gene, but they do it one step later on in the gene to protein pathway than methylation or acetylation does.
Back to our book example, it’s like if you have a cookbook (the DNA). You copy out a recipe on a piece of paper for later use (the RNA). Then you use the recipe to make cookies (the protein). Methylation puts big rocks in front of the bookshelf so you can't get to it and get at the cookbook. Acetylation glues the pages of the book together so you can’t read it. But microRNAs are your obnoxious husband who draws in marker all over your copied recipe, so you have to go back and copy it out again. (Disclaimer: while my husband is quite capable of being obnoxious, he has never defaced any of my recipes. He has scribbled notes on the medication list for my dogs in the face of my express requests to the contrary, however. Rosie hasn’t been on ciprofloxacin for six months but it still says “cipro” on her meds list. It’s like he’s incapable of thinking ahead.)
There is a
lot we don’t know about microRNAs. The whole epigenetics field is like this: we are getting to the point where we can detect these changes, but we still don’t really know what they mean. So in this study, they found that 9 microRNAs were expressed at different levels in sperm of the stressed mice versus the unstressed mice. We can make some predictions, using computer algorithms, about which messenger RNAs these microRNAs were going to stick to and silence, but we don’t know for sure that that’s what they were actually going to do.
Still, the predicted list is pretty interesting, because it contains the messenger RNA for the enzyme which controls methylation. Methylation! Another epigenetic mechanism! So is there some epigenetic chain going on here? The dad passes on microRNAs which will result in the DNA of the offspring being more or less methylated. It’s so hard to know what that means, because methylation has very different effects depending on which gene is affected, and this change is a more global change. But it’s a really intriguing finding, isn’t it?
Conclusions
This study is exciting, but I still felt a bit of disappointment as I read it. No behavior changes? Really? Is it really significant without the behavior changes? I mean, do we really
care about stress system changes if there are no behavior changes? Of course we do, and I wonder if future studies will investigate different behaviors, or behaviors at different points in the mouse’s life, and then we’ll understand this system a little better.
What does it mean for dogs? Of course it is immediately applicable to the question: if a male dog is stressed, will this stress affect his offspring? The answer is a nice solid maybe. In some way that we can’t really predict or define.
But at another level, this is another step in our progress towards understanding how genes and the environment interact. Stressful situations change gene expression in the stressed individual and possibly their offspring. How, why? How can we measure it? How can we use our knowledge to help an animal who has been traumatized, or undersocialized? Watching the field of epigenetics unfold is so much fun: everything is new, we understand so little, but the new technologies are coming so fast that we’re learning more and more.
![By Lilly_M [GFDL (http://www.gnu.org/copyleft/fdl.html) or CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEhLNCx0nKQxFVlsdWpji0DZ1s2jjFW1sYht4mOV40Af8-No961pcrdmEUnuNiU3UC7vUGo2wrs4Qsk8eLxnyWvNBQHH_XtKeMInEOFr9igaahtt-KenIXqwVMQ_4vZOUDtIQZ63Ry2DcxU/s320/96_well_PCR_plate-06.jpg)